PRODUCTS
| Product Name | Strength | Image (not to scale) | Pack Size | NDC # | RLD / Brand Name | TE Rating | Therapeutic Category |
|---|---|---|---|---|---|---|---|
| Fenofibrate Tablets | 54mg |
 |
90 |
68180-362-09 |
Lofibra® |
AB |
Antihyperlipidemic agent |
| Fenofibrate Capsules | 43mg |
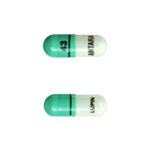 |
30 |
68180-0130-06 |
Antara® |
AB |
Antihyperlipidemic |
| Flucytosine Capsules USP | 250 mg |
 |
100 |
43386-771-01 |
Ancobon® |
AB |
Antifungal |
| Fluocinonide Topical Solution USP | 0.05% |
 |
20 mL |
43386-026-02 |
Lidex® |
AT |
Corticosteroid |
| Fluoxetine Tablets USP | 60mg |
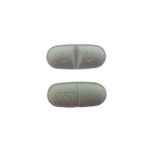 |
30 |
68180-997-06 |
Fluoxetine Tablets |
AB |
Anti-depressant |
| Fluoxetine Tablets USP | 10mg |
 |
30 |
68180-998-01 |
Prozac® |
AB |
Anti-depressant |
| Fyavolv™ | 0.5mg/0.0025mg |
 |
28 x 3 |
68180-827-09 |
FemHRT® |
AB |
Hormone Replacement Therapy |
| GaviLAX (PEG3350) Powder for Oral Solution | 17gm/scoopful |
 |
238g |
43386-312-14 |
MiraLAX® |
AA |
Laxative |
| GaviLyte™ – C | 240 -5.84-2.98-6.72-22.72gm |
 |
4 L |
43386-060-19 |
Colyte® |
AA |
Hyperosmotic |
| GaviLyte™ – G | 236-5.86-2.97-6.74-22.74 gm |
 |
4 L |
43386-090-19 |
GoLYTELY® |
AA |
Hyperosmotic |
| Hydrocortisone Butyrate Lotion | 0.1% |
 |
59 mL |
68180-951-01 |
Locoid® |
AB |
Corticosteroid |
| Hydrocortisone Valerate Cream USP | 0.2% |
 |
15 g |
68180-954-01 |
Westcort® |
AB |
Anti-inflammatory |
| Imipramine Pamoate Capsules | 75mg |
 |
30 |
68180-314-06 |
Tofranil-PM® |
AB |
Antidepressant |
| Jencycla™ | 0.35mg |
 |
28 x 3 |
68180-0877-73 |
Ortho-Micronor® |
AB2 |
Oral Contraceptive |
| Kaitlib™ | 0.8mg/0.025mg |
 |
28 x 3 |
68180-0903-73 |
Generess® |
AB |
Oral Contraceptive |
| Kurvelo™ | 0.15mg/0.03mg |
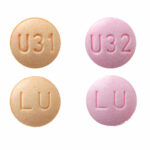 |
28 x 3 |
68180-0844-73 |
Nordette® |
AB |
Oral Contraceptive |
| Lamivudine and Zidovudine Tablets | 150mg/300mg |
 |
60 |
68180-0284-07 |
Combivir® |
AB |
Antiretroviral agent |
| Lanthanum Carbonate Chewable Tablets | 500mg |
 |
2 x 45's |
68180-819-42 |
Fosrenol® |
AB |
Phosphate Binder |
| Levetiracetam Tablets | 250mg |
 |
60 |
68180-113-02 |
Keppra® |
AB |
Anticonvulsant |
| Levetiracetam ER Tablets | 500mg |
 |
60 |
68180-117-07 |
Keppra® XR |
AB |
Antiepileptic |
| Levonorgestrel and Ethinyl Estradiol Tablets USP (0.1 mg/0.02 mg) and Ethinyl Estradiol Tablets USP (0.01 mg) | 0.1mg/0.02mg & 0.01mg |
 |
91 x 2 |
68180-0848-13 |
LoSeasonique® |
AB |
Oral Contraceptive |
| Levonorgestrel & Ethinyl Estradiol Tablets USP | 0.15mg/0.03mg |
 |
91 x 3 |
68180-0843-13 |
Seasonale® |
AB |
Oral Contraceptive |
| Levonorgestrel and Ethinyl Estradiol Tablets USP | 0.1mg/0.02mg |
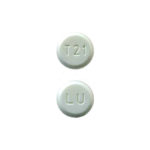 |
28 x 3 |
68180-0854-73 |
Lutera® |
AB1 |
Oral Contraceptive |
| Levonorgestrel and Ethinyl Estradiol Tablets USP | 0.05 mg/0.03 mg; 0.075 mg/0.04 mg and 0.125 mg/0.03 mg |
 |
28 x 3 |
68180-0857-73 |
Trivora® |
AB |
Oral Contraceptive |
| Levothyroxine Sodium Tablets USP | 25mcg |
 |
90 |
68180-970-03 |
Levoxyl® |
AB |
Thyroid hormone replacement |
| Lisinopril Tablets USP | 2.5mg |
 |
100 |
68180-981-03 |
Zestril® |
AB |
ACE Inhibitor |
| Lisinopril/HCTZ Tablets | 10/12.5mg |
 |
100 |
68180-518-01 |
Prinzide® |
AB |
ACE Inhibitor with Diuretic |
| Losartan Potassium Tablets USP | 25mg |
 |
30 |
68180-376-06 |
Cozaar® |
AB |
Angiotensin II Receptor antagonist |
| Losartan Potassium and Hydrochlorothiazide Tablets | 50/12.5mg |
 |
30 |
68180-215-06 |
Hyzaar® |
AB |
Angiotensin II Receptor antagonist w/ Diuretic |
| Lovastatin Tablets | 10mg |
 |
60 |
68180-467-07 |
Mevacor® |
AB |
HMG Coa Reductase Inhibitor |
| Mefenamic acid Capsules | 250mg |
 |
30 |
68180-0185-06 |
Ponstel® |
AB |
NSaID |
| MemAntine HCl Tablets USP | 5mg |
 |
60 |
68180-229-07 |
Namenda® |
AB |
NMDa Receptor antagonist |
| Memantine Hydrochloride Extended-Release Capsules | 7mg |
 |
30 |
68180-246-06 |
Namenda XR® |
AB |
Central Nervous System Agent |
| Metformin HCl ER Tablets | 500mg |
 |
60 |
68180-336-07 |
Fortamet® |
AB |
Biguanide Antihyperglycemics |
| Metformin Hydrochloride Extended-Release Tablets USP | 500mg |
 |
90 |
68180-338-01 |
Glumetza® |
AB |
Antihyperglycemic |
| Mibelas™ | 1 mg/0.02 mg |
 |
28 x 3 |
68180-0911-73 |
Minastrin® 24 Fe |
AB |
Oral Contraceptive |
*All registered trademarks are the property of their respective owners. These products are intended for U.S. residents only.
