
Our Founder, Dr. Desh Bandhu Gupta, was a firm believer that science was a trigger to enhancing health and empowering communities. DBG, as Dr. Gupta was fondly called, founded Lupin in 1968, and soon expanded the scope to producing life-saving drugs for Tuberculosis. This early pivot towards serving patient needs has instilled in us the commitment to focus on good health for communities globally. Today, we take pride in carrying on this legacy by prioritizing innovation, quality, and accessibility of our healthcare solutions. We focus on key therapeutic areas such as cardiovascular health, tuberculosis, diabetes, respiratory, gastrointestinal disorders, and women’s health. We are serving over 1,420,000 patients in 100+ countries through our patient support programs.
Our core values drive us, in our endeavor to fulfil our mission to deliver life changing medicines that meet diverse patient needs globally. Our patient first approach is: we focus on developing medical products for specific therapeutic areas and actively engage in addressing all stages of the healthcare cycle, from prevention to diagnosis and rehabilitation.
We are united by our Purpose – ‘To improve our patients’ lives’.
Our aspiration is to transform Hope into Healing for patients globally through cutting-edge science and technology, supporting the needs of our patients, colleagues, and communities, while delivering sustainable returns and creating value for everyone. We are guided by our four strategic priorities as we move forward to being an innovation-led transnational pharmaceutical company, dedicated to improving patient outcomes and advancing healthcare worldwide.
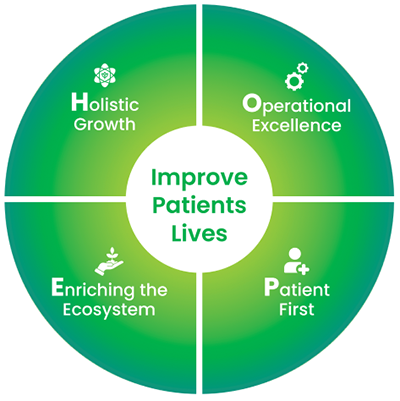
Our people are our greatest assets, and as our business evolves, we focus on business growth and value creation by developing a future ready workforce.
We are focused on efficiency extraction and productivity optimization to achieve excellence in our operations across different aspects of the business. This helps us streamline operations and maximize productivity.
We strive to provide patients with meaningful healthcare experiences and outcomes, helping them to lead fulfilling lives. Our decisions are driven by what patients identify as most important to them.
In India, we aim to surpass market growth by expanding our sales force in existing therapeutic areas of dominance such as Cardiology, Pulmonology and Diabetes Management and diversifying our portfolio to include Gynecology, Dermatology, Urology, and Pediatrics to serve a broader patient base.
In the U.S., we focus on first-to-market launches of complex generics, including biosimilars. In the EMEA region, we strengthen our leadership in the Respiratory and Neurology segments.
We are creating new platforms in inhalation, injectables, and biosimilars to expand our offerings and enhance complex dosage forms beyond oral solids.Lupin has always been a pioneer in technology adoption for advancing patient care. To enhance our connect with patients, we are working on advanced Digital and Diagnostic Solutions.
Lupin Diagnostics, our trusted network of labs and collection centers, is accessible to patients for their pathology needs. Lupin’s Lyfe solution is the first and only evidence-based Digital Therapeutics Solution for cardiac rehabilitation. Lupin is revolutionizing healthcare through apps such as Fight TB for TB patients and LegalRx for medico legal information for healthcare professionals. We are also advancing AI in healthcare with our AI chatbots, such as Anya and SAHAYAK. Atharv Ability, Lupin’s first Neurological Rehabilitation Center, located in Mumbai, serves as a cutting-edge outpatient facility for adults and children, for neurological rehabilitation.We work to ensure equitable access to medicines and healthcare worldwide, accelerating ARV and anti-TB registrations in low and middle income countries.
Through our partnership with the Tutudesk Foundation in South Africa, we aim to promote equitable access to education for 20 Mn children by 2025.Innovation is the cornerstone of all our initiatives and is a core competency. We relentlessly pursue excellence through continuous improvements in all our projects, processes, and products. We continue to focus on developing innovative products that address medical needs and enhance patient outcomes.
Our strategy includes expanding the inhalation pipeline with accelerated development and green propellant programs and establishing an injectables growth strategy for early success.We are committed to enriching the ecosystem by fostering strong collaborations with diverse stakeholders. By engaging with our suppliers and vendors and assessing their performance, including ESG compliance, we ensure mutual growth and adherence to high standards. We support healthcare professionals through continuous education and resources, enhancing their ability to provide exceptional patient care.
| Enriching the Ecosystem | |
|---|---|
|
Support to Local Communities |
Through the Lupin Foundation, we have established a sustainable, adaptable model of holistic rural development in India, serving over 1,405 villages across eight states in the year FY24. |
|
De-risking Value Chain |
high-value products, we engage with multiple suppliers, maintain buffer supplies, and utilize supply chain modeling to anticipate disruptions. Strategic investments in business intelligence and forecasting systems have enabled us to build a resilient global supply chain and ensure high service levels. We maintain consistent supplies by identifying and onboarding alternate vendors for critical APIs and intermediates. We also work with our vendors by assessing their adherence to our ESG principles. We are in the process of building their capabilities for a sustainabile value chain. |
|
Regulatory Compliance |
We ensure compliance with all applicable norms and regulations of national and international regulatory bodies. Additionally, our operations adhere to internationally recognized standards and certifications. These include environmental management, occupational health and safety, quality management, pharmaceutical quality systems, and laboratories for testing and calibration. |