BALTIMORE–(BUSINESS WIRE)– Lupin Pharmaceuticals, Inc. is voluntarily recalling 5 lots of Ceftriaxone for Injection, USP, 250mg, 10 lots of Ceftriaxone for Injection, USP, 500mg, 24 lots of Ceftriaxone for Injection, USP, 1g and 3 lots of Ceftriaxone for Injection, USP 2g, to the hospital/physician level. The products have been found to contain visual grey particulate matter in reconstituted vials.
Improper piercing and use of a needle greater than 21 gauge (larger internal diameter), while reconstituting the vial, can push rubber flecks into the solution. There were no grey flecks seen prior to the reconstitution of the vials and the issue was identified upon standard visual inspection prior to patient administration.
If injected, this product (containing rubber particulate matter from the stopper) could cause vein irritation/phlebitis or pulmonary embolic events that could result in permanent impairment of body function or damage to body structures, such as the lungs and vascular system. In addition, as ceftriaxone can be administered intramuscularly, the use of the product may result in local muscle inflammation and/or abscesses.
Ceftriaxone for Injection, USP, is used as a sterile, semi-synthetic, broad-spectrum cephalosporin antibiotic for intravenous or intramuscular administration. It is used to reduce the development of drug-resistant bacteria and maintain the effectiveness of ceftriaxone sodium and other antibacterial drugs Ceftriaxone for Injection, USP, should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. To date, the Company has not received any reports of adverse events related to the recalled lots.
Ceftriaxone for Injection, USP, is packaged in a glass vial, in pack of 10, containing 10 vials in a carton, with NDC
68180-611-10, 68180-622-10, 68180-633-10, 68180-644-10 and as single pack containing one glass vial in a carton with NDC 68180-611-01, 68180-622-01, 68180-633-01.
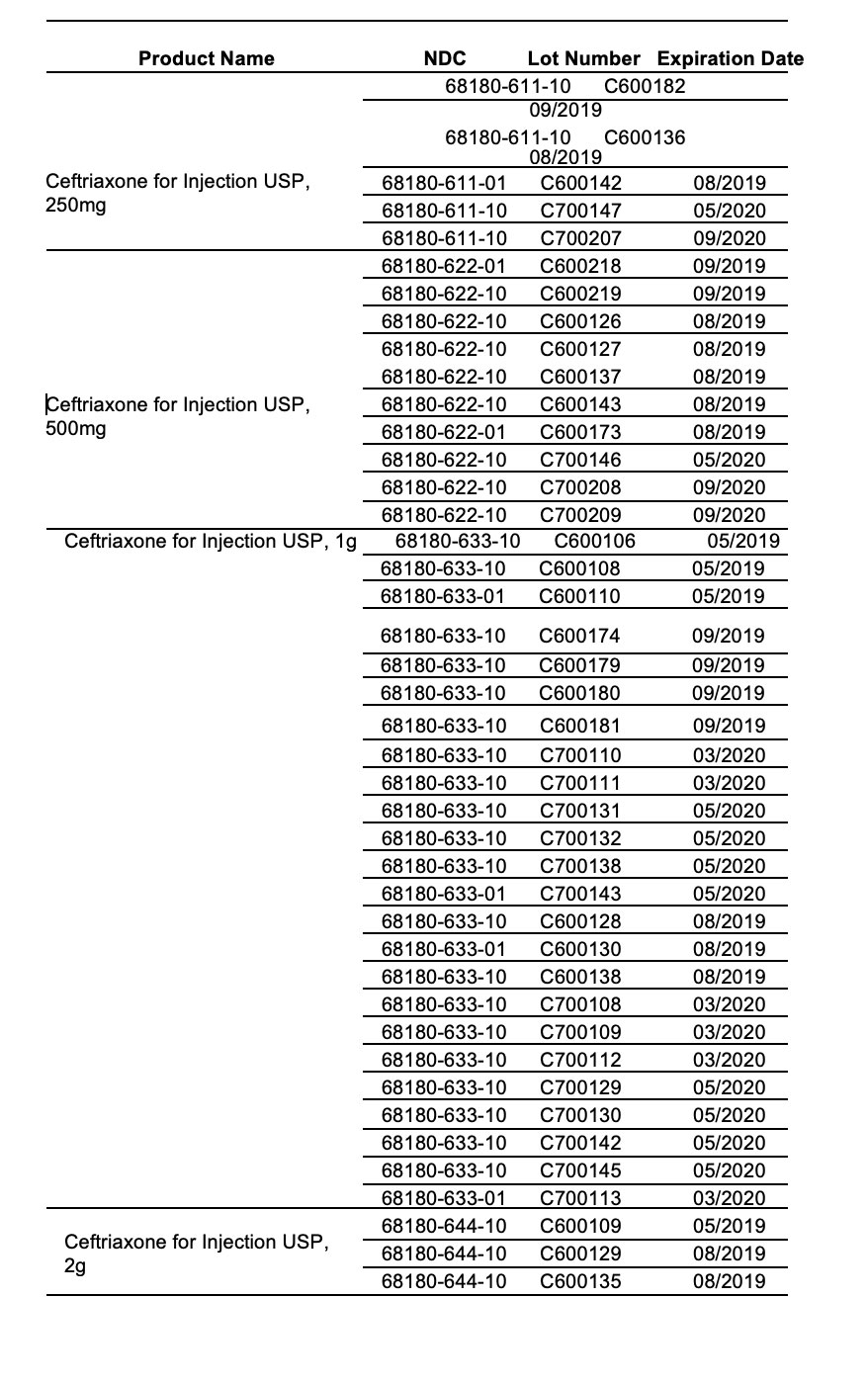
The lots of Ceftriaxone for Injection USP, 250mg, 500mg, 1g and 2g included in the recall are listed in the table below:
Ceftriaxone for Injection, USP, 250mg, Ceftriaxone for Injection, USP, 500mg, Ceftriaxone for Injection, USP, 1g and
Ceftriaxone for Injection, USP, 2g were distributed Nationwide to Wholesalers / Drug chains.
Lupin Pharmaceuticals Inc. is notifying its distributors by phone and through recall notification and is arranging for return of all recalled product lots.
Hospitals / Physicians that have Ceftriaxone for Injection, USP, which are being recalled should stop using and return to Genco Pharmaceuticals Services “a subsidiary of FedEx Supply Chain” 6101 North 64th Street, Milwaukee, WI 53218, Tel: (855) 838-5786.
Questions regarding this recall can be made by contacting GENCO Pharmaceutical Services at 1-855-838-5786
Monday – Friday 7:30 am to 6:00 pm EST. For reimbursement, please have the recalled lots returned to GENCO, the lot number can be found on the side of the vial. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch
Adverse Event Reporting program either online, by regular mail or by fax.
Complete and submit the report Online: www.fda.gov/medwatch/report.htm1
Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm2 or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800- FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.